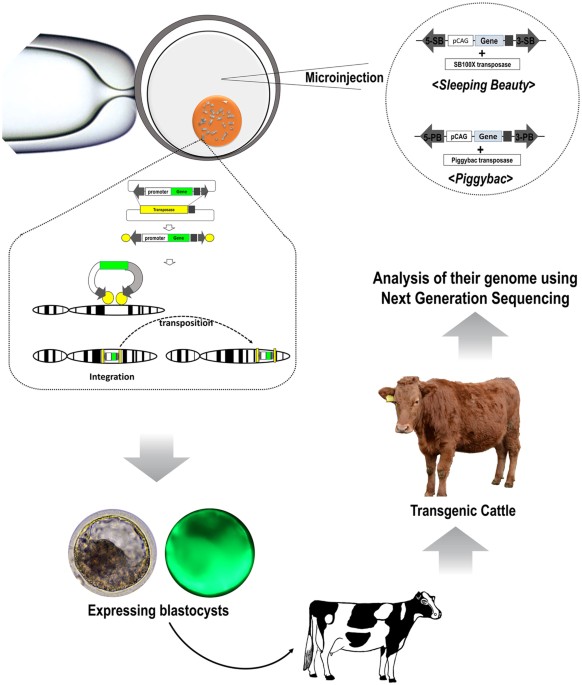
In a groundbreaking development at the intersection of biotechnology and agriculture, researchers have achieved a significant milestone: the creation of the world’s first transgenic cow capable of producing human insulin in her milk. Led by scientists from the University of Illinois Urbana-Champaign and the Universidade de São Paulo, this pioneering endeavor holds immense promise for revolutionising insulin production, potentially alleviating drug scarcity and reducing costs for millions of individuals living with diabetes worldwide.
Published in the Biotechnology Journal, the study describes a proof-of-concept achievement that could reshape the landscape of diabetes treatment. By harnessing the natural capabilities of the mammary gland, researchers have unlocked the potential to produce insulin—a life-saving hormone—in a cost-effective and sustainable manner.
Professor Matt Wheeler, the lead author of the study, underscores the significance of leveraging nature’s design to address pressing healthcare challenges. “Mother Nature designed the mammary gland as a factory to make protein really, really efficiently. We can take advantage of that system to produce a protein that can help hundreds of millions of people worldwide,” explains Wheeler.
The groundbreaking research involved the insertion of a segment of human DNA encoding proinsulin—the precursor to active insulin—into the cell nuclei of cow embryos. Through meticulous genetic engineering techniques, the researchers ensured that human DNA was targeted for expression specifically in mammary tissue, thereby minimizing the presence of human insulin in other tissues or the bloodstream.
Upon the birth of a transgenic calf, the team observed the production of human proinsulin and insulin in the cow’s milk—an unexpected but welcome outcome. “The cow basically processed it herself. She makes about three to one biologically active insulin to proinsulin,” notes Wheeler, highlighting the remarkable efficiency of the mammary gland in producing functional insulin.
While the initial lactation yielded smaller quantities of milk than anticipated, the potential for scalable production remains promising. Wheeler estimates that a single cow could produce significant amounts of insulin, potentially meeting the demands of millions of individuals with diabetes.
The research team aims to enhance the efficiency and yield of insulin production by refining their techniques and optimizing the reproductive process in transgenic cows. By establishing purpose-built herds of transgenic cattle, the researchers envision a future where insulin production can be seamlessly integrated into existing agricultural practices.
The implications of this research extend far beyond the realm of diabetes treatment. With the potential to harness the mammary gland’s protein production capabilities, transgenic cows could pave the way for the sustainable production of a wide range of therapeutic proteins and pharmaceuticals.
However, significant hurdles remain before transgenic cow-derived insulin can be widely adopted. Regulatory approval, infrastructure development, and scaling up production are among the challenges that must be addressed. Nonetheless, Wheeler remains optimistic about the future of transgenic cow technology.
“I could see a future where a 100-head herd, equivalent to a small Illinois or Wisconsin dairy, could produce all the insulin needed for the country,” he envisions. “And a larger herd? You could make the whole world’s supply in a year.”
As research continues to advance at the intersection of biotechnology and agriculture, the prospect of affordable and accessible healthcare solutions grows ever closer. With transgenic cows leading the charge, the future of insulin production and diabetes treatment looks brighter than ever before.