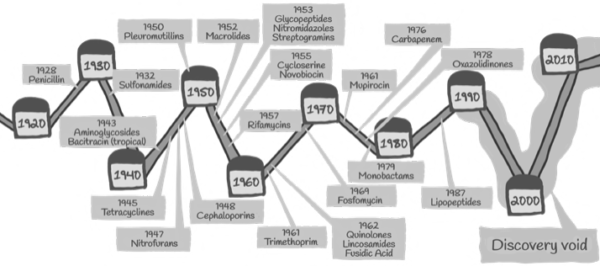
In the realm of antibacterial discovery, the emergence of novel classes of antibiotics represents a significant milestone in the ongoing battle against bacterial infections. Against a backdrop of increasing antibiotic resistance and dwindling treatment options, the discovery of gepotidacin—a first-in-class triazaacenaphthylene antibacterial—by Vanderbilt biochemists and their collaborators at GlaxoSmithKline (GSK) heralds a new era in antibacterial therapeutics. This article delves into the groundbreaking research led by Neil Osheroff and his team, shedding light on gepotidacin’s mechanism of action, efficacy against urinary tract infections (UTIs), and potential impact on clinical practice.
Unraveling the Mechanism of Action
Gepotidacin’s journey from laboratory discovery to potential clinical application is anchored in a comprehensive understanding of its mechanism of action. Through meticulous analysis, Vanderbilt researchers elucidated gepotidacin’s dual targeting of gyrase and topoisomerase IV—key enzymes involved in bacterial DNA replication and repair—in Escherichia coli, the primary causative agent of UTIs. This unique mode of action, characterized by simultaneous inhibition of two essential enzymes, represents a formidable barrier to the development of resistance—a critical advantage in the fight against antibiotic resistance.
Clinical Implications: A Beacon of Hope for UTI Treatment
UTIs, affecting 50%–60% of women in their lifetime, represent a significant burden on public health, necessitating effective antibacterial interventions. Gepotidacin’s promising efficacy against UTIs, demonstrated in preclinical and clinical studies, positions it as a valuable addition to the antimicrobial arsenal. Notably, outcomes from clinical trials, including EAGLE-2 and EAGLE-3, underscore gepotidacin’s efficacy and safety profile, offering hope for patients with UTIs refractory to conventional antibiotics. The prospect of a new class of antibacterials approved for UTI treatment marks a paradigm shift in clinical management, offering clinicians a much-needed alternative for patients with limited therapeutic options.
Navigating Challenges and Opportunities
As gepotidacin inches closer to regulatory approval, attention turns to the challenges and opportunities associated with its clinical implementation. Vigilant surveillance for emerging resistance and strategic health economic analyses will be pivotal in optimizing gepotidacin’s use and preserving its efficacy in the long term. Moreover, ongoing research endeavors aimed at elucidating gepotidacin’s pharmacokinetic properties, drug-drug interactions, and potential applications in other infectious diseases hold promise for further expanding its clinical utility.
Paving the Way Forward
In a landscape characterized by dwindling antibiotic pipelines and escalating antimicrobial resistance, gepotidacin’s emergence as a novel antibacterial class offers a glimmer of hope in the quest for effective infectious disease management. The collaborative efforts of academic researchers and industry partners have propelled gepotidacin from bench to bedside, underscoring the transformative potential of interdisciplinary collaboration in drug discovery. As gepotidacin embarks on its journey toward regulatory approval and clinical adoption, its success promises to reshape the treatment landscape for UTIs and pave the way for future innovations in antibacterial therapeutics. With steadfast commitment and strategic foresight, gepotidacin stands poised to make a profound impact on global health by combatting bacterial infections and safeguarding human well-being for generations to come.