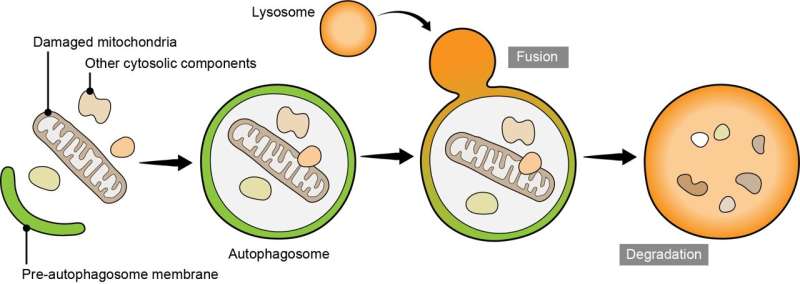
The intricate dance of cellular processes orchestrates the delicate balance of life within our bodies, ensuring that each organelle plays its part in maintaining cellular health. Among these choreographed movements lies the phenomenon of autophagy, a cellular recycling system crucial for removing damaged organelles and cellular debris. Within the realm of autophagy, a specific pathway known as mitophagy takes center stage, tasked with the selective removal of dysfunctional mitochondria—a process intricately linked to the pathogenesis of Parkinson’s disease.
In a recent breakthrough study published in The EMBO Journal, researchers from Tokyo Medical and Dental University TMDU have shed light on the molecular intricacies underlying mitophagy, unraveling the pivotal role of an enzyme called Tank-binding kinase 1 TBK1 in orchestrating this essential cellular process. Led by Koji Yamano and his team, the study elucidated the intricate interplay between TBK1 and an autophagy adaptor protein called optineurin OPTN in the context of mitophagy mediated by Parkinson’s disease-associated proteins PINK1 and Parkin.
Parkinson’s disease, a debilitating neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons, has long been associated with defects in mitochondrial function and quality control mechanisms. Central to this pathology are PINK1 and Parkin, proteins implicated in the selective targeting of damaged mitochondria for degradation via mitophagy. Yamano and his colleagues embarked on a quest to unravel the molecular underpinnings of TBK1’s involvement in this disease-relevant mitophagy pathway.
Through a series of meticulous experiments employing molecular biology techniques, the researchers uncovered a intricate network of protein interactions essential for the initiation and execution of mitophagy. They found that TBK1 plays a crucial role in facilitating the association of OPTN—an autophagy adaptor protein—with damaged mitochondria marked by ubiquitin chains catalyzed by PINK1 and Parkin. This interaction between OPTN and TBK1 at the mitophagy contact sites is pivotal for the activation of TBK1 via autophosphorylation—a prerequisite for its function in mitophagy.
Moreover, the researchers employed innovative techniques to validate their findings, including the generation of specific molecules called monobodies designed to inhibit the physical interactions between OPTN and its binding partners. Through the inhibition of OPTN accumulation at mitophagy contact sites, the researchers effectively blocked TBK1 activation and subsequent mitochondrial degradation—a compelling demonstration of the critical role played by the OPTN-TBK1 axis in regulating mitophagy.
The implications of these findings extend far beyond the realm of basic research, offering tantalizing prospects for the development of novel therapeutic interventions for Parkinson’s disease. By elucidating the molecular mechanisms governing mitophagy, researchers may pave the way for the development of targeted drugs aimed at restoring mitochondrial homeostasis and alleviating the neurodegenerative symptoms associated with Parkinson’s disease.
As we delve deeper into the molecular intricacies of mitophagy and its role in neurodegenerative disorders, the potential for groundbreaking discoveries and therapeutic breakthroughs becomes increasingly apparent. The study conducted by Yamano and his team represents a significant step forward in our understanding of Parkinson’s disease pathogenesis, offering new insights into potential therapeutic targets and paving the way for future research endeavors aimed at combating this devastating condition.
The unraveling of the molecular dance of mitophagy represents a triumph of scientific inquiry, illuminating the intricate mechanisms that underpin cellular homeostasis and disease pathology. As we continue to decipher the molecular mysteries of the cell, we inch closer to unlocking the secrets of Parkinson’s disease and ushering in a new era of targeted therapies and personalized medicine.