Endometriosis, a condition where tissue similar to the lining of the uterus grows outside the womb, affects millions of women worldwide. Its complex etiology has long puzzled researchers, but recent advancements in genetic analysis offer promising avenues for understanding its origins. Among the emerging frontiers is the investigation of the gut microbiome’s influence on endometriosis development.
In a groundbreaking study published in BMC Women’s Health, researchers employed a bidirectional two-sample Mendelian randomization analysis to explore the causal relationship between gut microbiota and endometriosis. Led by Chunxiao Dang and colleagues, the study represents a pivotal step towards unraveling the intricate interplay between microbial composition and this debilitating gynecological disorder.
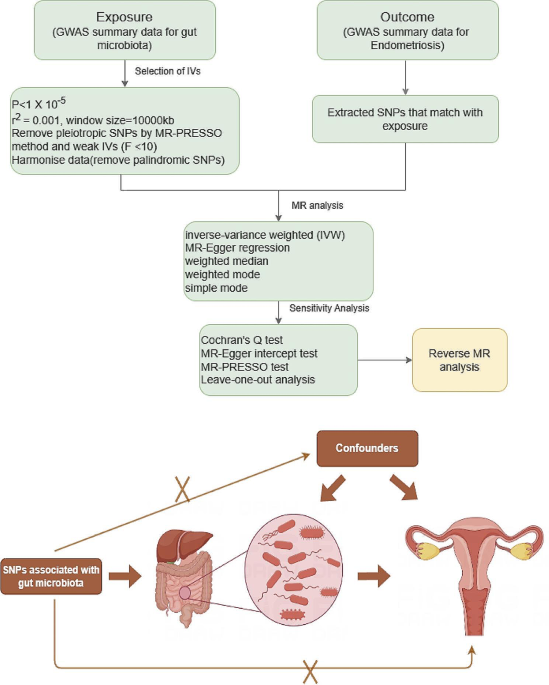
The study delves into the burgeoning field of Mendelian randomization, a methodological approach that leverages genetic variants as instrumental variables to infer causal relationships between exposures and outcomes. By utilizing genome-wide association study (GWAS) data from large cohorts, researchers identified significant associations between specific gut microbiota taxa and endometriosis risk.
The findings shed light on several key microbial players implicated in endometriosis pathogenesis. Among the noteworthy associations uncovered were the identification of certain microbial taxa as risk factors, including Prevotellaceae, Anaerotruncus, Olsenella, Oscillospira, and Bacillales. Conversely, protective effects were attributed to Melainabacteria and the Eubacterium ruminantium group.
Notably, the study’s rigorous methodology included comprehensive sensitivity analyses to assess the robustness of the results. Heterogeneity testing, pleiotropy evaluation, and sensitivity analyses all corroborated the validity of the observed associations, lending credibility to the causal relationship between gut microbiota and endometriosis risk.
The implications of these findings extend beyond elucidating the pathophysiology of endometriosis. By uncovering microbial taxa associated with disease risk, the study paves the way for the development of novel diagnostic and therapeutic strategies. Harnessing the insights gleaned from genetic analyses, clinicians may one day be able to tailor interventions targeting the gut microbiome to mitigate endometriosis progression and improve patient outcomes.
Moreover, the study underscores the intricate interplay between the gut microbiome, immune function, and estrogen metabolism in endometriosis. Dysbiosis within the gut microbiota may disrupt immune homeostasis and exacerbate inflammatory responses, perpetuating the pathological processes underlying endometriosis. Additionally, the gut microbiome’s influence on estrogen metabolism further underscores its role as a potential therapeutic target in endometriosis management.
While the study represents a significant leap forward in our understanding of endometriosis, further research is warranted to elucidate the mechanistic underpinnings of the observed associations. Future investigations may explore the dynamic interplay between the gut microbiome and endometrial tissue, shedding light on the bidirectional communication between these two compartments.
The study offers compelling evidence supporting a causal relationship between gut microbiota and endometriosis risk, providing a foundation for future research endeavors. By unraveling the intricate web of interactions between microbial composition and disease pathogenesis, researchers may unlock new avenues for precision medicine approaches in endometriosis management. As our understanding of the gut microbiome continues to evolve, so too does the promise of transformative breakthroughs in women’s health.